5% RU58841 (50mg/ml)
60 ml per unit (Largest size on the market)
The raw material is >99% pure. See purity test report here.
Best price & best quality. Formulated by professionals who have been pioneering in developing various solutions for the unmet needs.
New on June 30, 2022: Now PG+ethanol based version is available. This product is named RuDerma. The previously launched version was formulated without PG (propylene glycol), called RuDerma PG Free.
If you ordered RuDerma before June 30, 2022, what you ordered was RuDerma PG Free. It is a version free of propylene glycol (PG), which is an excellent alternative to the KB solution based Ru 58841.
Both versions can be selected from the production options (next to the price on this page).
Free Product Offer: You can request a free sample of this product (20 ml) if you never ordered RuDerma before. The link for requesting a free sample is provided at the end of FAQ on this page.

What is RU58841?
It is also known as RU, RU-58841, PSK-3841 or HMR-3841, and is a nonsteroidal anti-androgen (NSAA). It is also one a common SARMs (Selective Androgen Receptor Modulators). Its structure is shown below. It is nonsteroidal because it does not have the steroidal skeleton structure. It was investigated and developed by ProStrakan (a Scottish based specialty pharmaceutical company) for its potential therapeutic applications in conditions that were driven by androgens. Conditions, such as acne, hirsutism (excessive hair growth), and androgenetic alopecia (male-pattern hair loss). ProStrakan conducted two clinical studies, phase I and II, and the results of 5% RU-58841, when applied twice daily, were reported to be similar to finasteride.

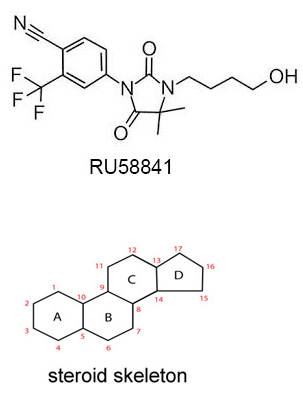

How does it work?
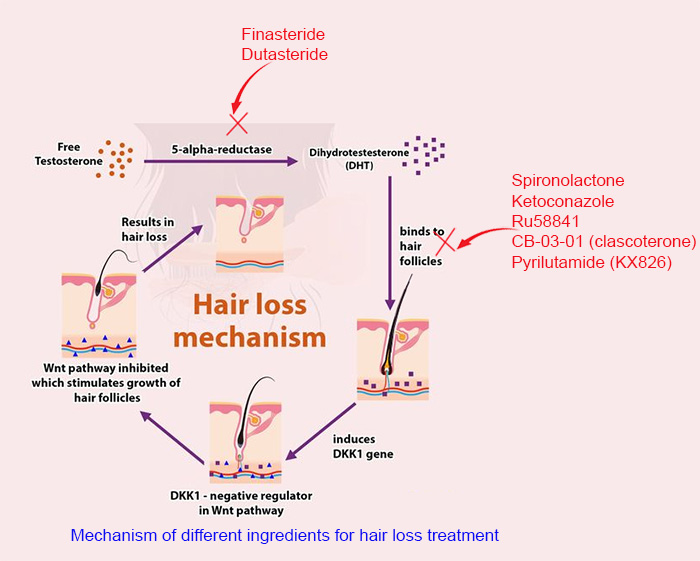
As a non-steroidal anti-androgen, RU binds itself to androgen receptors and directly inhibits the effects of endogenous (from our own body) androgens such as testosterone and dihydrotestosterone (DHT). As a first generation non-steroidal anti-androgen, it selectively competes for the androgen receptors. This competition effectively attenuates the activity of testosterone and DHT, leading to an overall anti-androgen effect.
The way it works is similar to that of spironolactone, and ketoconazole, a key ingredient used in hair loss shampoo.
It differs from finasteride or dutasteride, which inhibits the activity of 5α-Reductase, an enzyme that converts testosterone into dihydrotestosterone (DHT). DHT is the primary hormone that causes hair loss in men. See the figure below.
Based on the mechanism, it is apparent that finasteride and dutasteride may lead to a decreased DHT level, while RU58841 does not. DHT is pivotal to a male's sexual functionality, that is reason why some people choose RU instead of finasteride. This is also why more people are using topical finasteride instead of oral form to avoid its systemic effect (lowering DHT on the scalp instead of the whole body).
Topical RU-58841 solution has a relatively short half-life (1 hour) in comparison to Finasteride (6 hrs) and minoxidil (4 hrs).
How do you control the quality of the product?
Each batch is tested by a 3rd party. Only material that is tested to be >99% pure will be used in our product. (See the test reports here).
How long does one unit last?
The product is for research use only. Only the user knows how long one bottle will last. One unit is 60 ml (not 30 ml) of 5% RU58841 (i.e. 50 mg/ml). There is 3 grams of RU in one unit.
Can I mix RuDerma with a minoxidil solution?
RuDerma can be mixed with any of our minoxidil products. They are miscible to each other and the mixture is stable for at least 2 months. This product can be mixed with other liquid minoxidil of different brands, such as Kirkland.
How to use RuDerma?
The product is for research purposes only. It is for in vitro studies and laboratory usage only. It is not for human consumption. We buy our materials from reliable sources and confirm the purity and identity after we receive them. Ru58841 used in RuDerma is >99% pure.
What is the solvent composition?
We can't disclose the exact composition of the solvent because it is our trade secret. However, we can say that the regular RuDerma has propylene glycol and ethanol (aka, alcohol) and RuDerma PG Free has glycerin and ethanol.
How stable is RU58841?
There is conflicting information about the stability of the RU premixed solution. Some say it is stable for years even when it is stored at room temperature, while others say it is only stable for up to 2 weeks. The truth probably lies somewhere in between.
If the solution was stable for only two weeks, it is unlikely that ProStrakan would even conduct the phase I and II clinical trials. The project must have been killed long before that.
Stability of RU58841 powder: RU powder is stable for at least two years when stored in an airtight container in a freezer.
RU58841 Side Effects
Unfortunately, there are no phase III clinical trials for this material. Hence, there is no publicly available longitudinal data concerning its side-effect and safety profile in humans. Indeed, the last known study to have verified its efficacy and performance was published in 2005 [Munster, U., et al., RU5-8841-myristate--prodrug development for topical treatment of acne and androgenetic alopecia. Pharmazie, 2005. 60(1): p. 8-12.]. No studies in the literature have been scoped to identify any risks and side-effects of this product. However, the side-effects can be extrapolated based on its classification as a non-steroidal anti-androgenic compound.
Simply speaking, RU doesn't decrease DHT levels. However, it hinders the function of DHT. Thus, it may cause similar sexual side effects caused by the deficiency of DHT.
It is worth noting that someone who gets side effects from finasteride or dutasteride may not necessarily get side effects from RU, and the opposite is true.
RU58841 vs finasteride
As mentioned above, RU58841 and finasteride all work by disrupting the DHT pathway. Fin decreases the production of DHT, while RU prevents DHT from binding to androgen receptors. Both substances may lead to similar side effects because they all affect the function of DHT. Some people think topical RU may have an advantage over fin because it is more of local effect, having less systemic sides than 5a-redutase inhibitors such as fin.
Most people prefer use fin because its safety profile is well established by many clinical trials on human subjects and it is FDA approved to treat hair loss. At the same time, RU never had a phase III clinical study. In addition, fin is well tolerated by most people. The chance of having fin side effects is low. If topical finasteride is used, the possibility of having side effects is even lower. Nonetheless, if someone is super sensitive to fin or dut and absolutely can't tolerate them in any form, is RU an option then?
RU58841 vs minoxidil
Minoxidil is the no.1 hair loss treatment. It is FDA approved to treat hair loss for since 1988. It is an antihypertensive vasodilator.
The mechanism by which minoxidil promotes hair growth is not fully understood. In theory, it widens blood vessels and opens potassium channels, allowing more oxygen, blood, and nutrients to the follicles. In addition, minoxidil contains a nitric oxide moiety and may act as a nitric oxide agonist. This may cause follicles in the telogen phase to shed, which are then replaced by thicker hairs in a new anagen phase.
One thing for sure is that minoxidil does not affect DHT level or its function. Therefore, it won't cause any sexual side effects.
Do you ship to my country?
We ship worldwide, including all European countries. We will cover the VAT and duty taxes when we ship to European and any other countries.
What happens if my package gets lost?
We will reship your order if your package gets lost during the shipping. More shipping related questions can be found here.
How to request a free sample of RuDerma?
If you never ordered RuDerma before, you are eligible for a free sample of RuDerma (20 ml). You can request the free sample here.
There have been several studies on the performance, efficacy and safety of RU5881 since 1994 when it was first developed by French researchers. In the original study by Battmann et al. (1994), it was found to display a high and extremely specific binding to the androgen receptors in mice. Compared to other non-steroidal anti-androgens such as flutamide, RU had a staggering 30-fold higher affinity for the androgen receptors. Importantly, Battmann and his fellow researchers found that the local activity of RU-58841 was significant at an extremely low dose (1µg/animal), but was limited to the application site. This meant that the anti-androgenic effects of it were limited to the target area where it was applied. Indeed, the researchers found that RU- 58841 did not exert any anti-androgenic effects on deep accessory sex organs or the serum (blood) testosterone level.
Four years later, American researchers tested RU-58841 in macaques (a type of monkey) [1]. They found that the topical application of this substance resulted in a significant increase in density, thickening and length of hair in macaques with androgenetic alopecia. However, similar to the 1994 study, they did not observe any adverse systemic effects. This observation led them to conclude that RU was a promising therapeutic modality for patients with androgen-driven conditions such as acne, hirsutism and androgenetic alopecia. These findings echoed observations made by Japanese researchers in a similar study – 20 bald scalps of macaques were subjected to Finasteride and RU[2].
Another group of researchers examined 20 scalp grafts from men with androgenetic alopecia; these were grafted onto mice and monitored every month for six months [3]. Ten scalp grafts were each subjected to the RU-588 41 topical solution and ethanol (as a control) respectively. Although both groups had similar (28 vs 29) active follicles which appeared in total, the RU-58841 group demonstrated a second growth cycle more so than the ethanol group (8 vs 2). This led the researchers to conclude that RU- 58841 led to a higher linear hair growth rate and had therapeutic potential for androgenetic alopecia [3].
Studies to date have been limited to studies performed on cultured cell lines in Petri dishes and animal studies. ProStrakan did perform phase I (5% topical solution administered twice daily over 4 weeks to a total of 30 people.) and phase II clinical trials (5% and a 2.5% topical solution administered once a day over six months in 120 people.) of the drug on humans to evaluate its safety in 2002 and 2003. The results of those trials were not published.
The information from animal studies indicates that the action of RU should be localized to where it is applied.
1. Pan, H.J., et al., Evaluation of RU5884 1 as an anti-androgen in prostate PC3 cells and a topical anti-alopecia agent in the bald scalp of stumptailed macaques. Endocrine, 1998. 9(1): p. 39-43.
2. Obana, N., C. Chang, and H. Uno, Inhibition of Hair Growth by Testosterone in the Presence of Dermal Papilla Cells from the Frontal Bald Scalp of the Postpubertal Stumptailed Macaque1. Endocrinology, 1997. 138(1): p. 356-361.
3. De Brouwer, B., et al., A controlled study of the effects of RU58 841, a non-steroidal antiandrogen, on human hair production by balding scalp grafts maintained on testosterone-conditioned nude mice. Br J Dermatol, 1997. 137(5): p. 699-702.
PRODUCT REVIEW
CUSTOMERS WHO VIEWED THIS PRODUCT ALSO VIEWED
Reviews